Screening of veterinary drug residues in products of animal origin
purpose
Provide an effective solution for the screening of more than 150 veterinary drug residues in animal-derived products.
Foreword
Veterinary drugs are widely used in the treatment or prevention of animal diseases, but will bring trace drug residues in animal products such as meat, fish, milk, eggs and honey. Due to the potential harm to human health, the existence of drug residues in the food chain has also attracted widespread attention. Indeed, contaminated animal products may cause allergic reactions or indirect problems in clinical treatment due to the development of bacterial resistance. In order to protect the health of consumers and ensure the quality of animal products, the maximum allowable content of drugs in animal products, that is, maximum residues (MRLs) 1-3 has been established worldwide.
Traditionally, residues of veterinary drugs in biological materials have been analyzed by microorganisms and immunohistochemical techniques4. These methods can provide a quick and economic screening method for specific classes of compounds. Each test kit is generally only effective for one compound, lacking selectivity for the identification of fuzzy substances, and the results are only approximately quantitative. As for positive results, government regulations often require more accurate chromatographic methods to determine the presence and content of antibiotics.
As MRLs regulations become more stringent, it is more important to develop quantitative methods and confirmation and identification techniques to minimize false positive results. Time-of-flight mass spectrometry (TOF MS) screening has certain advantages, such as historical data tracking, simplified instrument method settings, and no need to compromise on the effectiveness of the method when increasing the number of compounds, so it is widely welcomed. However, processing and reviewing TOF screening data usually involves a complex workflow. When the positive peak is first identified, the consumer risk is then quantified. The conversion from qualitative to quantitative processes is usually done manually. It sets up an obvious data inspection resource channel and a high error rate.
This application example describes Waters® ACQUITY UPLC® and quadrupole time-of-flight Xevo ™ QTof mass spectrometry for more than 150 veterinary drug residues and metabolites, including avermectins, benzimidazoles, β-agonists, β- On-site screening of lactams, corticosteroids, macrolides, nitroimidazoles, quinolones, sulfonamides, tetracyclines and other veterinary products. ACQUITY UPLC separation analysis is faster, while maintaining high efficiency and resolution. ACQUITY UPLC is combined with Xevo QTof mass spectrometry to maintain high-sensitivity separation efficiency, detection selectivity, and accurate quality. The data is processed by POSI ± IVE ™ software, so that accurate quality data can be qualitative and quantitative in a simple channel, and important quantitative results are directly obtained for positive test substances.
experiment
Sample Preparation
Previous reports have described sample preparation methods for milk4. For liver, blood, fish, and meat samples, 5g samples were used with 20 mL acetonitrile and 5 g anhydrous Na2SO4. After centrifugation, 0.4 mL of DMSO was added to 4 ml of supernatant. Dry acetonitrile, add 0.8 g of water to the original concentration of the sample, and centrifuge before injection. Appendix 1 lists the veterinary drug residues screened.
UPLC conditions
LC system: ACQUITY UPLC
Column: ACQUITY UPLC BEH C18
1.7 μm, 2.1 x 100 mm
Column temperature: 40? C
Mobile phase A: 0.1% formic acid (anhydrous)
Mobile phase B: acetonitrile + 0.1% formic acid
Gradient: Time (min)% A
0.00 95
0.25 95
6.00 5
7.00 5
7.20 95
9.00 95
Flow rate: 0.40 mL / min
Injection volume: 20 μL full loop
MS conditions
MS system: XEVO QTof MS
Acquisition mode: MSE
Ion mode: ESI positive capillary pressure: 2.4 kV
Cone hole voltage: 30 V
MS collision energy: 6 V
MSE collision energy range: 25 to 35 V
Ion source temperature: 120 ℃
Desolvation temperature: 400 ℃
Desolvated gas flow rate: 800 L / hr
Conical air flow: 20 L / hr
Acquisition range: m / z 50 to 1000 for 0.1 s
Xevo QTof mass spectrometry (mass calibration using sodium formate and locked mass detection) is automatically set up using IntelliStart â„¢ software.
Data acquisition and processing
The data was obtained with Waters MassLynx ™ version 4.1 and processed with POSI ± IVE software. In MSE acquisition mode, data is always collected through two channels: low collision energy (CE) for molecular ion information; and high CE for product ions. Figure 1 shows the TOF screening workflow. 
Figure 1. TOF screening workflow, the theoretical steps from sample preparation to data analysis, including integrated instruments and software to ensure that the process runs seamlessly
Results and discussion
The selectivity for complex materials comes from the high chromatographic resolution of ACQUITY UPLC and the high mass spectral separation rate of Xevo QTof mass spectrometry and its ability to reproduce the mass spectrum within a narrow mass window. Figure 2 shows the total ion current chromatogram (TIC) of danofloxacin in bovine liver extract and its corresponding accurate mass spectrum, using this as an example. 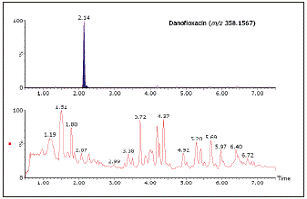
Figure 2. The total ion current chromatogram (TIC) of danofloxacin in bovine liver and its corresponding rustic map. The high resolution is very helpful for the selectivity and credibility of the obtained results. However, in some cases, additional information, such as product ions, may produce significant benefits for the uncertain compound being tested. Figure 3 shows the increased credibility in the monitoring results of xylazine and molentyl. The precise mass (m / z 221.1112) and elemental composition (C12H16N2S) of the two substances are the same, and the retention times are similar (2.44 and 2.52 min). Xylazine and Molentyl product ions are the same (m / z 164.0536), but can be identified by the only product ions specified (m / z 90.0366 and 150.0381). Very high mass spectrometry resolution will not be able to separate these substances, so structural information is very important for the fuzzy identification of residues to be detected.
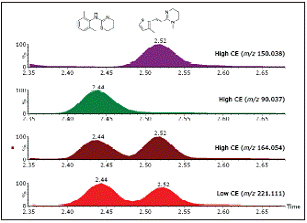
Figure 3. Clear identification of residues with the same exact mass and the same, Xylazine (2.44 min) and Molentyl (2.52 min) under MSE
Product ion information can be obtained by in-source fragmentation, MS / MS or MSE:
In-source cleavage is a way to directly obtain product ions, but it is less sensitive than MS mode, and it is difficult to control due to ion source conditions with changes in mobile phase and matrix. MS / MS is a digital-based technology that can provide high-quality product ion spectra, but has a low duty cycle and shifts when high abundance remains, and must be used when the composition of the sample is known in advance. MSE is a patented digital-based technology that provides a simple, offset-free, parallel path to deliver accurate mass molecule (MS) and product ion (MSE) information for each detectable component without the need for multiple injections.This information is only valid when product ions can be detected at corresponding levels in the matrix. Figure 4 shows the mass spectrum of low collision energy and high collision energy of sulfadoxine in bovine blood extract in MSE mode, which proves that the product ions can be detected at the corresponding level in the matrix.
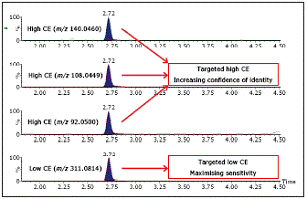
Figure 4. MSE mode mass spectra of sulfadoxine in bovine blood at low and high CE, showing that product ions can be detected at relevant levels in the matrix.
Figure 5 shows the difference between the spectra of low CE and high CE obtained in MSE mode. The low CE spectrum is dominated by molecular ions, while the high CE spectrum contains product ions, which increases the credibility of identification. 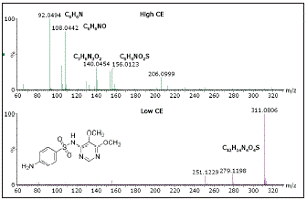
Figure 5. MSE pattern spectrum of sulfadoxine in bovine blood at low CE and high CE
Analyze the sample to obtain a total cycle time of 9 min (including equilibration time). This makes the actual sample processing capacity of more than 100 samples per day. However, this sample throughput and 150 residues per sample pose significant problems for data processing and calculation.
The POSI ± IVE software is specially developed to reduce the data calculation time for checking TOF mass spectrometry screening data, ensuring that only positives (accurate quality and retention time are within predetermined deviations) and temporary (retention time is acceptable, but marked as accurate (Quality exceeded) detection will be automatically quantified. Only a list A of target compounds containing the compound name, structural formula and retention time is required, and the length of this list is not limited.
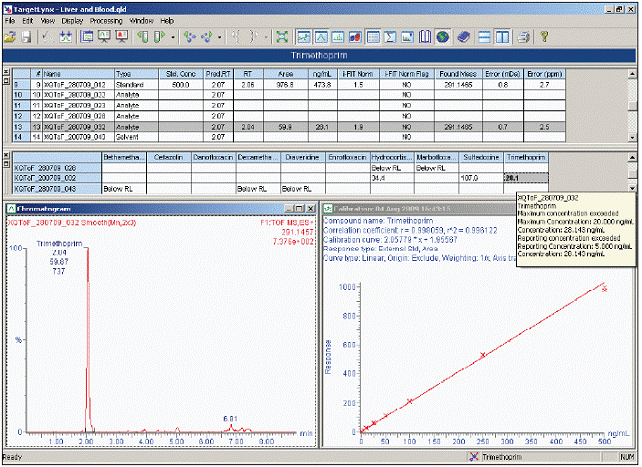
During the automatic calculation process, the POSI ± IVE software performs a qualitative search, using mass accuracy and retention time to determine whether the compound is positive, temporary, or not detected, to generate information on the presence / absence of the compound in the target list. All positive and temporary tests are then automatically quantified and reported using the TargetLynx browser report, with isotope composition (iFit ™) detection. Figure 6 shows the results of the POSI ± IVE Xevo QTof mass spectrometry screening of bovine blood extracts. Of the more than 150 veterinary drugs, only three were detected qualitatively and quantitatively: hydrocortisone, sulfadoxine, and trimethoprim. The test samples showed positive identification and quantitative results of trimethoprim in bleeding samples.
Since it is not included in the calculation method, hydrocortisone was not reported in the original directional analysis. One of the advantages of the POSI ± IVE semi-directional approach is that there is no limit to the length of the residue list, and there is no mandatory calibration standard. The recorded data file will be recalculated by the new information, and hydrocortisone will be detected and quantified in the original data file.
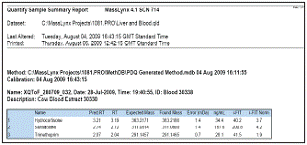
Figure 7. Bosi blood POSI ± IVE Xevo QTof MS screening report indicates the presence of 3 residues
Figure 7 A part of the report of the bovine blood extract generation, in which the range of 150 residues was narrowed down to 3 kinds of retention time, accurate molecular weight and iFIT identified as positive.
in conclusion
1. Due to its potential harm to human health, the existence of veterinary drug residues in the food chain has received widespread attention.
2. The ACQUITY UPLC and Xevo QTof mass spectrometry schemes simplify the screening of more than 150 veterinary drug residues in animal-derived products under appropriate MRLs.
3. When designating the identification of residues to be inspected, MSE information adds additional credibility and overcomes the limitations of conventional data-dependent approaches.
4. The information-rich nature of TOF MS data increases its requirements for data processing software, so reducing manual processing and automatically repeating tasks is necessary to improve the quality of results and the acceptance of TOF MS.
5. POSI ± IVE significantly reduces the bottleneck of the TOF mass spectrometry screening data processing by ensuring that only positive and temporary tests can be automatically quantified. The nature of automatic processing reduces the possibility of errors by eliminating manual transcription steps in the workflow.
references

Appendix 1:
MethylprednisoloneAlbendazole
Albendazole
Albendazole sulfoxide
Amoxicillin
Ampicillin
Azithromycin
Benzocaine
Betamethasone
Brombutrol
Bromhexine
Karalor
Carbados
Carbenicillin
Cefaclor
Cefadroxil
Cefalexin
Ceflonine
Cephalosporin
Cefazolin
Cefoperazone
Cefotaxime
Cefoxitin
Cefquinoxime sulfate
Cefsulodin
Ceftiofur
Ceftriaxone
Cefuroxime
Cephalosporin
Cephalothin
Cefpirin
Cefradine
Chlortetracycline
Sebutro
Sinofloxacin
Ciprofloxacin Clenbuterol
Kronpro
Clopidogrel
Cloxacillin
Cyclobendazole
Danofloxacin
Dapsone
Nortetracycline
Dexamethasone
Veratropine
Dicloxacillin
Ethylamine
Difloxacin
Metronidazole
Antelopecyl dinitroimidazole
Deoxytetracycline
Enoxacin
Enfloxacin
Erythromycin
Non-bantair
Fenbendazole
Fenbendazole sulfone
Fleroxacin
Flubendazole
Flubendazole
Flumequine
Flumequine
Hydrocortisone
Ipronidazole
Hydroxyisoprazole
Jiasamycin
Ketoprofen
Cylindromycin A1
Levamisole
Levamisole
Lomefloxacin Marbofloxacin
Mebendazole
5-hydroxybenzimidazole
2-amino-5-benzoylbenzimidazole
Meloxicam
Metronidazole
Hydroxy metronidazole
Minocycline hydrochloride
Morenthal
Morenthal
Nalidixic acid
Nalidixic acid
Natamycin
Norfloxacin
Novobiocin
Ofloxacin
Oquidose
Oleanmycin phosphate
Oxacillin
Oxfendazole
Orbendazole
Oxolid
Oxytetracycline Hydrochloride
Pefloxacin
Penicillin G
Penicillin v
Praziquantel
Prednisolone
Prednisolone
Promethazine
Pyrimethamine
Thiadiazine
Rifaximin
Ronidazole
Roxarone
Roxithromycin
Shu Chuan Ling
Safloxacin
Spiramycin-l
Sulfabenzoyl
Sulfaacetamide
Sulfachloropyrazine Sodium
Sulfachlordazine
Sulfadiazine
Sulfadesoxine
Sulfadoxine
Sulfathiazole
Sulfamidam
Sulfamethazine
Sulfamethoxine
Sulfamethazine
Sulfamethazine
Sulfamethoxazole
Sulfamethoxazole
Sulfamethoxine
Sulfaxazole
Sulfanitrobenzene
Sulfapyridine
Sulfaquinoxaline
Sulfathiazole
Sulfatraxazole
Sulfadiazine
Sulfaisoxazole
Tenidazole
tetracycline
Tetramazole
Thiabendazole
5-hydroxythiabendazole
Thiuram
Thiuram
Tilmicosin
Tolbutamide
Tolfenamic acid
Triclabendazole
Triclobendazole sulfone
Trichlorobendazole sulfoxide
Triflupromazine
Trimethoprim
Triacetyl oleandromycin
Toltrol
Taylor Star
Weimycin M1
Xylazine
Serenol
Glass Coffee Table,Glass Dining Table,Glass Table,Glass Desk
Hongsing Glass Hardware Porduct Co Ltd , https://www.hongsingglass.com